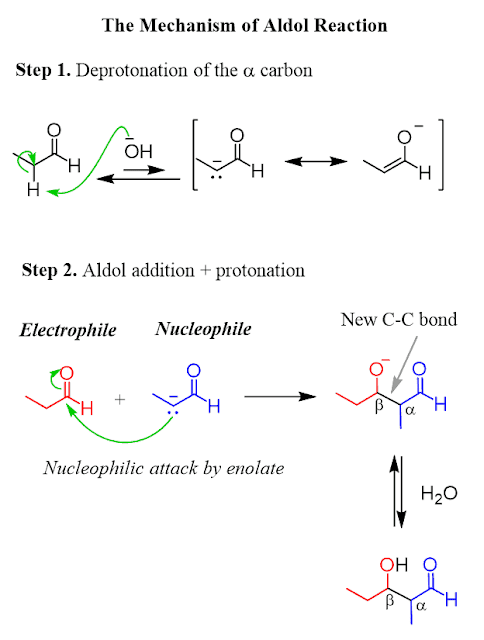
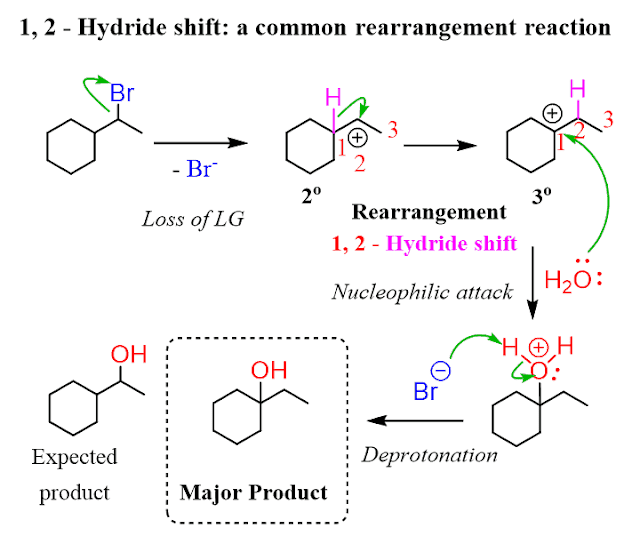
A Level H2 /H1 Chemistry tuition and notes by ex-JC teacher
Reach your full potential with A Level H2 / H1 Chemistry tuition by ex-JC teacher Why struggle in your chemistry studies? Do you need a grade boost? Do you want to do well for tests and exams? You’ve come to the right place! My personalized and customised A level H2 /H1 Chemistry tuition is now available in 2025. I was an ex-JC tutor, graduated with a NUS Honours degree in Pure Chemistry, along with years of solid experience. I also graduated with a diploma in education from NTU - NIE. I was the top student in a secondary school, achieved 5 distinctions in A levels in a top junior college, and I was in Dean's list in NUS. 1-1 individual tuition service is provided, with value added services to improve the students' grades. Individual attention to the student and customised tuition are given.Customised feedback with motivation tips are provided for the student. Ad-hoc tuition with topical revision is also available. My offer to...